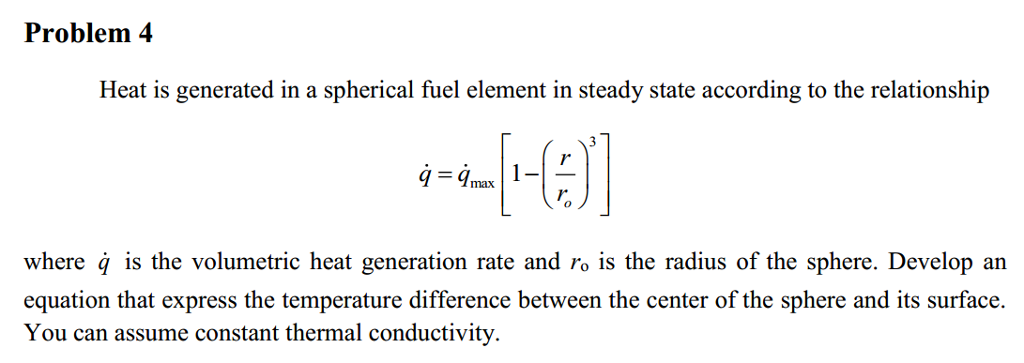
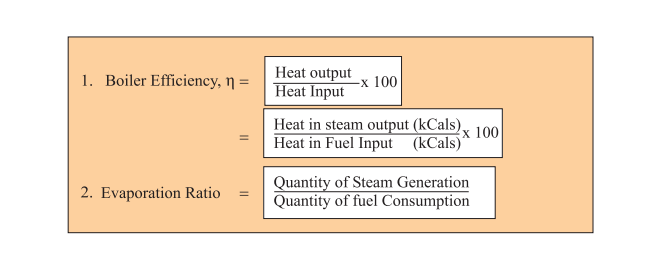
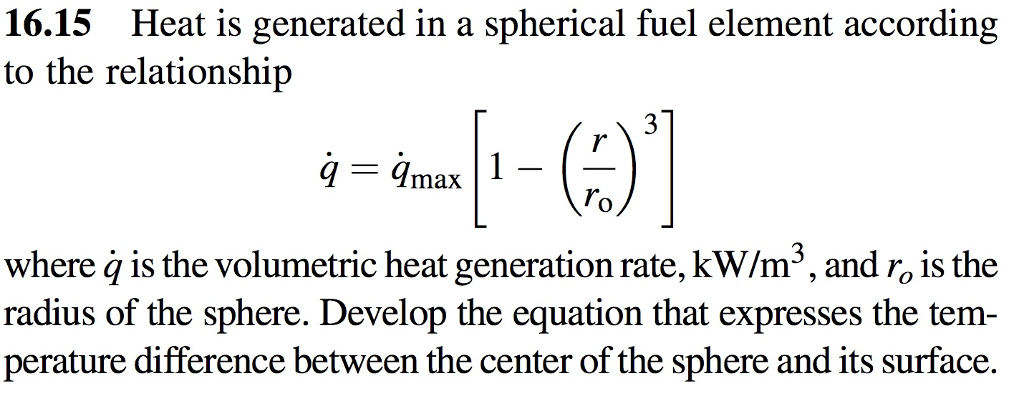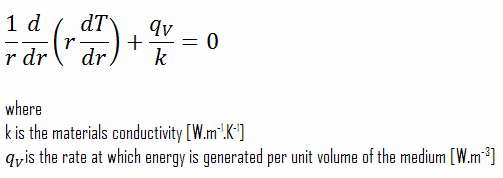In an experiment, 4.5 kg of a fuel was completely burnt. The heat produced was measured to be 180,00

In an experiment 4.5 kg of a fuel was completely burnt. The heat produced was measured to be 1,80,000 kJ. Calculate the calorific value of the fuel(in kJ/kg)?

In an experiment 4.5 kg of a fuel was completely burnt. The heat produced was measured to be 1,80,000 kJ. Calculate the calorific value of the fuel(in kJ/kg)?

Methane Pyrolysis for Zero-Emission Hydrogen Production: A Potential Bridge Technology from Fossil Fuels to a Renewable and Sustainable Hydrogen Economy | Industrial & Engineering Chemistry Research
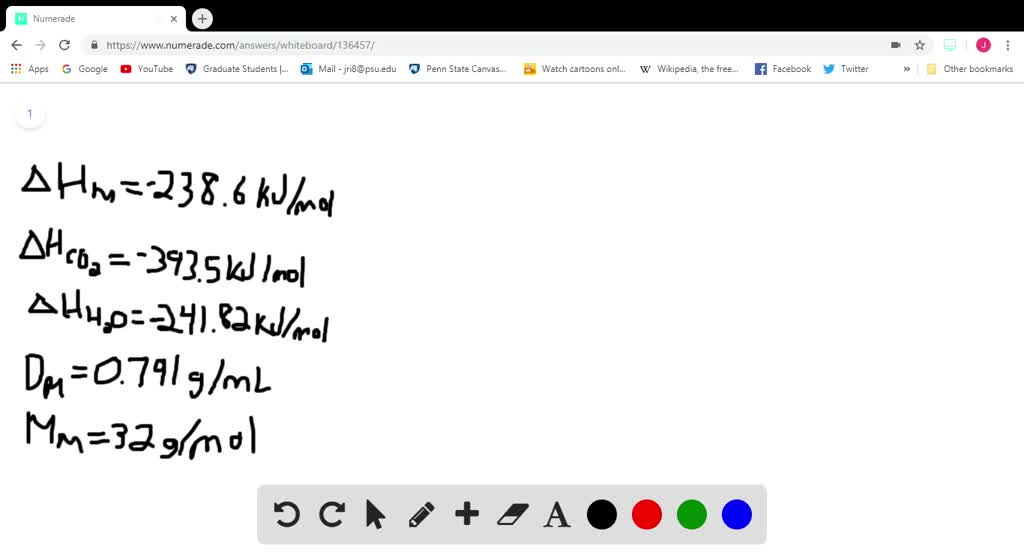
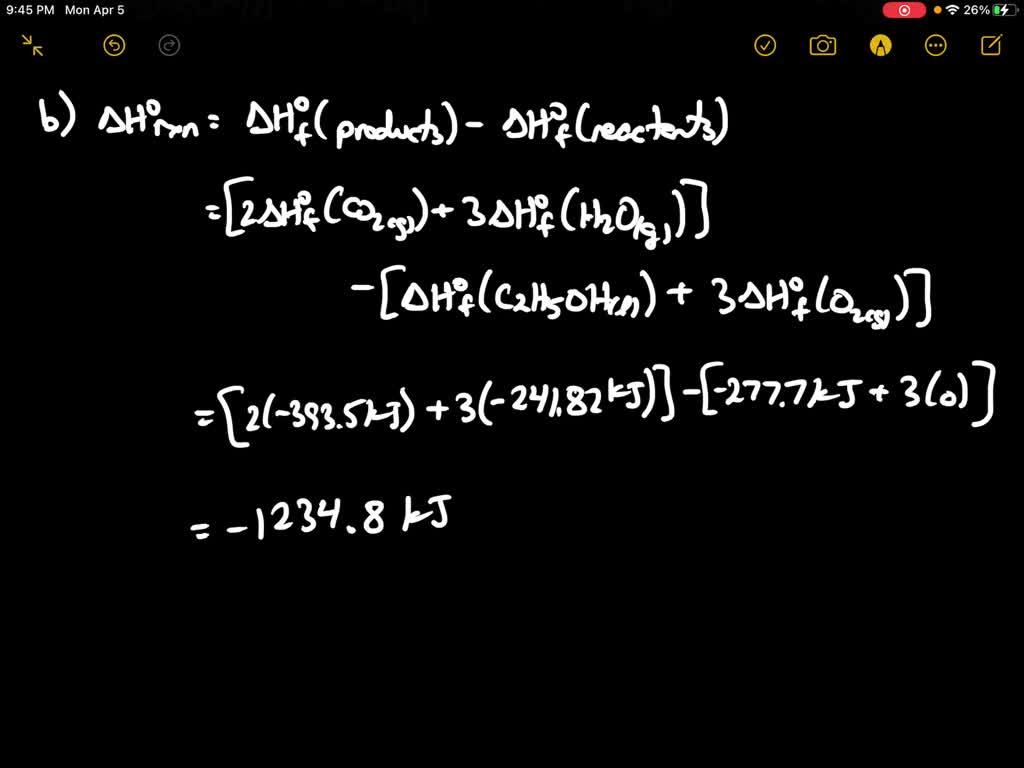
SOLVED:Methanol (CH3 OH) is used as a fuel in race cars. (a) Write a balanced equation for the combustion of liquid methanol in air. (b) Calculate the standard enthalpy change for the