![Calculate the mole fraction of NaOH in 10% w/w aqueous solution. [At. MassH=1, O=16, Na=23u] - Brainly.in Calculate the mole fraction of NaOH in 10% w/w aqueous solution. [At. MassH=1, O=16, Na=23u] - Brainly.in](https://hi-static.z-dn.net/files/da5/28dabad35d0225715bc998463bc22ff5.jpg)
Calculate the mole fraction of NaOH in 10% w/w aqueous solution. [At. MassH=1, O=16, Na=23u] - Brainly.in

Calculate the molarity of NaOH in the solution prepared by dissolving its 4 g in enough water to... - YouTube

A sodium hydroxide solution containing 40% by weight of pure NaOH has a specific gravity of 1.5. What volume of this solution will be required in the preparation of 500ml of a

How to prepare N/10 NaOH solution - Chemistry - Some Basic Concepts of Chemistry - 7762175 | Meritnation.com
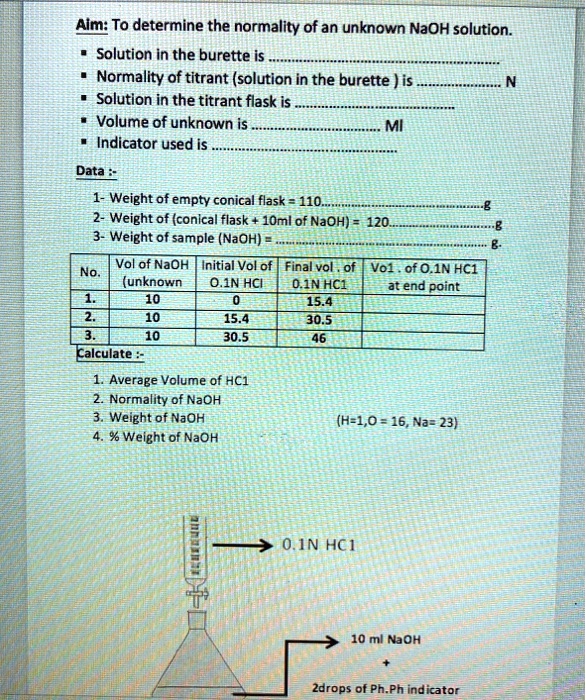
SOLVED: Aim: To determine the normality ofan unknown NaOH solution Solution in the burette is Normality of titrant (solution in the burette ) is Solution in the titrant flask is Volume of

100mL` of `10% NaOH(w/V)` is added to `100 mL` of `10% HCI(w/V)`. The nature of resultant solut... - YouTube

4 g NaOH is added in 100 mL of 0.5 M NaOH solution and solution was made 1 L with addition of water 20 mL of above solution can:

SOLVED: Calculate the pH of a 6.7 X 10-2 M NaOH solution. ( a strong base) ( Your answer should have 2 digits after the decimal)